Our laboratory aims to understand the fundamental scientific principles that regulate the pathogenesis of rheumatic diseases with the goal of developing novel medical interventions. We have a long-standing interest in immune mediated disorders, and study a broad range of modulatory signals that include immune responses, autophagy, aging, as well as genetic and environmental signals.
Osteoimmunology/Bone Biology/Arthritis
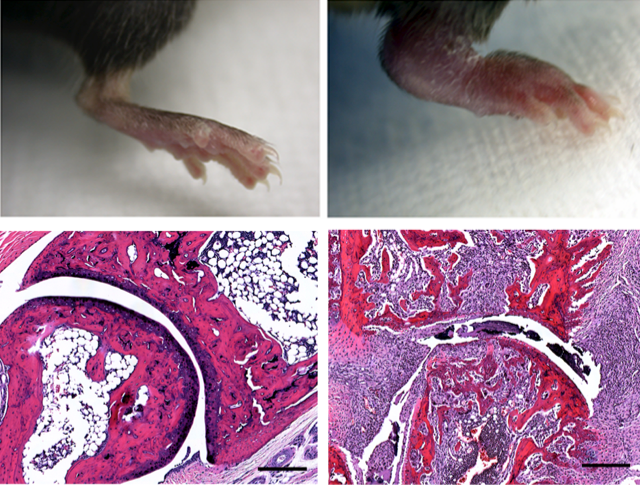
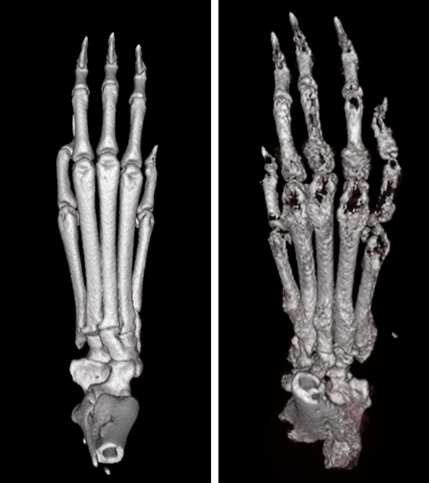
Bone destruction is hallmark feature of osteoporosis and commonly occurs in multiple rheumatic diseases such as rheumatoid arthritis, psoriatic arthritis and lupus. In our laboratory, we study the molecular mechanisms that control osteoclast differentiation and activation, the cells that are primarily responsible for bone resorption in rheumatic diseases. We have developed unique animal models of osteoporosis and animal models of inflammatory bone loss. We use these models to study the interaction of the immune and skeletal systems and identify novel biology that can be exploited therapeutically.
Projects:
1. The role of IL-23 in bone destruction
We have previously shown that IL-23 plays a critical role in osteoclast differentiation and activation. Specifically, IL-23 in vivo gene transfer induces myelopoiesis and osteoclast differentiation in a Th17 independent manner. We are translating this data using human osteoclasts isolated from peripheral blood mononuclear cells (PBMC’s), with ongoing efforts to further define the cellular and molecular events that orchestrate the responses elicited by IL-23.
2. Involvement of leukotriene B4 in Juvenile Arthritis (JA)
A critical issue in JA development that remains unresolved is how autoimmune processes are linked to a localized onset of inflammation in the joints. LTB4 is a potent proinflammatory mediator that causes inflammatory cell infiltration and also has direct effects on osteoclast differentiation and activation. The temporal correlation between the LTB4 generation and the onset of arthritis phenotype suggests that LTB4 may represent a critical link between autoimmune processes and the onset of joint inflammation. Therefore, targeting LTB4 synthetic or signaling pathways may represent a novel and effective therapeutic strategy for the treatment of JA. +
3. The IL-23/IL-17 axis in psoriatic arthritis
Some of the mechanisms that regulate osteoclast differentiation are also influencing other cell types causing a systemic inflammation. This inflammation results to clinical manifestations that closely resemble psoriatic arthritis and we have developed animal models to study the pathogenetic mechanisms. Psoriatic arthritis (PsA) is an autoimmune disease where the interaction of the immune and skeletal system often results in bone loss. We have previously shown that IL-17A upregulates the receptor RANK on human osteoclast precursors to increase their responsiveness to RANKL leading to increased bone loss in vitro. In this project, we are investigating the cellular and molecular interactions that lead to epidermal hyperplasia and arthritis as it's commonly seen in psoriatic arthritis. We developed recombinant mini-circle DNA carrying the IL-23, IL-17A and RANKL genes and evaluate the effects of mini-circle-mediated gene transfer in vivo.
4. Autophagy in skeletal development
Autophagy is a process by which the cell degrades its own components by first sequestering them to specialized vacuolar organelles, called autophagosomes and then fusing these vacuoles with lysosomes. Autophagy proteins have been shown to regulate the secretory component of osteoclastic bone resorption and TNF-mediated joint destruction in experimental arthritis. Therefore, the implications of autophagy in the pathogenesis of inflammatory arthritis may present us with new treatment avenues in combating inflammatory musculoskeletal diseases.
5. Collaborations with Industry
We partner with multiple biotech and pharmaceutical companies to develop novel inhibitors for the treatment of autoimmune disorders and to perform general discovery and mechanism of action projects. Our partnerships focus on strategies to develop biologic therapies for autoimmune disorders such as, osteoporosis, psoriatic arthritis, rheumatoid arthritis, inflammatory bowel disease, and psoriasis.
Osteoimmunology/Bone Biology/Arthritis
Bone destruction is hallmark feature of osteoporosis and commonly occurs in multiple rheumatic diseases such as rheumatoid arthritis, psoriatic arthritis and lupus. In our laboratory, we study the molecular mechanisms that control osteoclast differentiation and activation, the cells that are primarily responsible for bone resorption in rheumatic diseases. We have developed unique animal models of osteoporosis and animal models of inflammatory bone loss. We use these models to study the interaction of the immune and skeletal systems and identify novel biology that can be exploited therapeutically.
Projects:
1. The role of IL-23 in bone destruction
We have previously shown that IL-23 plays a critical role in osteoclast differentiation and activation. Specifically, IL-23 in vivo gene transfer induces myelopoiesis and osteoclast differentiation in a Th17 independent manner. We are translating this data using human osteoclasts isolated from peripheral blood mononuclear cells (PBMC’s), with ongoing efforts to further define the cellular and molecular events that orchestrate the responses elicited by IL-23.
2. Involvement of leukotriene B4 in Juvenile Arthritis (JA)
A critical issue in JA development that remains unresolved is how autoimmune processes are linked to a localized onset of inflammation in the joints. LTB4 is a potent proinflammatory mediator that causes inflammatory cell infiltration and also has direct effects on osteoclast differentiation and activation. The temporal correlation between the LTB4 generation and the onset of arthritis phenotype suggests that LTB4 may represent a critical link between autoimmune processes and the onset of joint inflammation. Therefore, targeting LTB4 synthetic or signaling pathways may represent a novel and effective therapeutic strategy for the treatment of JA. +
3. The IL-23/IL-17 axis in psoriatic arthritis
Some of the mechanisms that regulate osteoclast differentiation are also influencing other cell types causing a systemic inflammation. This inflammation results to clinical manifestations that closely resemble psoriatic arthritis and we have developed animal models to study the pathogenetic mechanisms. Psoriatic arthritis (PsA) is an autoimmune disease where the interaction of the immune and skeletal system often results in bone loss. We have previously shown that IL-17A upregulates the receptor RANK on human osteoclast precursors to increase their responsiveness to RANKL leading to increased bone loss in vitro. In this project, we are investigating the cellular and molecular interactions that lead to epidermal hyperplasia and arthritis as it's commonly seen in psoriatic arthritis. We developed recombinant mini-circle DNA carrying the IL-23, IL-17A and RANKL genes and evaluate the effects of mini-circle-mediated gene transfer in vivo.
4. Autophagy in skeletal development
Autophagy is a process by which the cell degrades its own components by first sequestering them to specialized vacuolar organelles, called autophagosomes and then fusing these vacuoles with lysosomes. Autophagy proteins have been shown to regulate the secretory component of osteoclastic bone resorption and TNF-mediated joint destruction in experimental arthritis. Therefore, the implications of autophagy in the pathogenesis of inflammatory arthritis may present us with new treatment avenues in combating inflammatory musculoskeletal diseases.
5. Collaborations with Industry
We partner with multiple biotech and pharmaceutical companies to develop novel inhibitors for the treatment of autoimmune disorders and to perform general discovery and mechanism of action projects. Our partnerships focus on strategies to develop biologic therapies for autoimmune disorders such as, osteoporosis, psoriatic arthritis, rheumatoid arthritis, inflammatory bowel disease, and psoriasis.